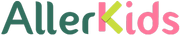Life, Health, Hope: Advancements and Updates in Research and Care in Food Allergies.
Allerkids .com @ 2017-12-17 11:51:18 -0800FARE NATIONAL FOOD ALLERGY CONFERENCE
Saturday May 16, 2016

Dr. James Baker, CEO and Chief Medical Officer of Food Allergy Research & Education.
I stepped out of a five hour flight from San Francisco into the balmy tropics of Orlando Florida, and walked straight into a room full of parents at the Hyatt Regency Hotel. I'm here for the FARE National Food Allergy Conference, a gathering of the country’s leading food allergy experts and members of the food allergy community for a weekend of world-class exchanges.
The Friday evening session was conducted by Jennifer Kurko, Founder and CEO of Kiss Freely Natural Cosmetics. The instant camaraderie amongst the mothers exchanging personal stories during Jennifers’ session was very touching.

I had so much to take in, and with the time conversion to Eastern, I could barely wind down to go to sleep. Regardless, I woke up the next morning energized and looking forward to learning more. Day one, I found myself facing the TV screen on my bathroom mirror, NEWSFLASH, it screamed, you have three minutes to get ready! AHhhh.

I zipped down the elevator to the breakfast hall and grabbed a quick 20 minute plate of fruit and muffin, simultaneously introducing myself to, and chatting with, parents that I had just met. When the clock stroke 8 am, we walked into what was quickly becoming a full house, commencing the first session of the day.

The FARE conference kicked off with an introduction from CEO and chief medical officer, Dr. James R. Baker, Jr.
The session was titled, ‘Life, Health, Hope: Advancements and Updates in Research and Care’.
Dr. Baker spoke about a ‘breakthrough‘ new drug by the name of Dupilumab(by Regeneron and Sanofi), that has proven safe and effective in treating both asthma, and atopic dermatitis.
Dr. Baker went on to discuss Food Allergy therapies in Clinical development. These included the DBV-Viaskin Patch and Aimmune Oral Immunotherapy. He stated that the key to pushing these therapies to the point of breakthroughs, was to define a path for FDA approval. This can only be done, he said, by facilitating large scale phase III trials. In order to do the latter, we need to increase overall sites of clinical trials for food allergies.
In 2015 FARE launched a program called the ‘Fare Clinical Network‘ (FCN), with a goal to increase clinical trial sites in the US and internationally; as well as facilitate the development of patient-centric research programs; and establish a National Advisory Board.
There are currently twenty four FCN sites operating, with the hope of adding another four to five sites in 2016; and a plan to grow to 40–50 local and international clinical trial sites.
The goal is to get enough patients into clinical trials that will give the numbers and results required for FDA approval.
Electronic medical and health records will also be put in place, so that data can be accessed and shared electronically.
Dr. Baker mentioned that the FCN currently costs $2.5 million per year, but will have an estimated cost of $6.5 million per year upon expansion. As such, FARE will need to work with the clinical trial sites to make the program sustainable.
Next, Dr. Panida Sriaroon, MD, Associate Professor of Pediatrics at the University of South Florida Morsani college of Medicine, took to the stage. Dr. Sriaroon is also Associate Director of the Allergy/Immunology fellowship training program, and the outpatient USF/Johns Hopkins All children’s hospital Allergy/Immunology clinic.
She also has an 18 month old son who has peanut allergies.

Dr. Sriaroon commenced by pointing out what we currently know about food allergies.
- Food allergies effect 8% of children under 3 years of age.
- There is a 2% increase in the prevalence of peanut allergies.
- Advances in the field include new biomarkers, new diagnostic tools, and new therapies.
- There is no FDA approved treatment for food allergies. The only treatment that is followed for food allergies, is strict food avoidance, and emergency treatment of using epinephrine auto-injectors, followed by a rush to ER if required.
She then went on to discuss what we don’t know about food allergies.
- We don’t know why there has been a significant increase in food allergies, in the last 20 years.
- We don’t know what the genetic versus environmental factors are that impact the prevalence of food allergies.
- We don’t know what the best diagnostic approaches are to identify food allergies.
- We don’t know what the best treatment options are for food allergies, nor whether we are closer to a cure, and personalized medicine.
She then mentioned that the good news is that there are key advances in food allergy research studies, and these include:
- Advances in prevention; the early peanut consumption study or LEAP/LEAP-ON, which is a clinical trail investigating how to prevent peanut allergy. LEAP answered the questions, should young children eat peanuts or avoid them? Which is a better approach to preventing peanut allergy?
- Advances in diagnostic testing; allergen component–resolved diagnostic testing (CRD). This method is able to determine risk of the severity of allergic reactions in specific cases, like soy, peanut, and hazelnut allergy.
- Advances in treatment such as allergen specific immunotherapy which includes, Food Oral Immunotherapy (OIT), Food Sublingual Immunotherapy (SLIT) and Epicutaneous Immunotherapy (EPIT).
- The use of biologics, which are selective immune antagonists for the treatment of allergic diseases. Dupilumab, a biologic that binds to IL-4Rα and blocks the Th2 cytokines IL-4 and IL-13 activity has been successful in clinical trials for the treatment of asthma and atopic dermatitis. There may be value in investigating applications of biologics for the treatment of food allergy.
She mentioned that some questions that prevail on the LEAP study include:
- Will the LEAP study work in non-high risk kids the same way it does for high-risk allergy prone kids?
- How much of the allergen does the child have to eat to maintain tolerance?
- Can the findings from the LEAP study be applied to other foods like milk, eggs and tree nuts?
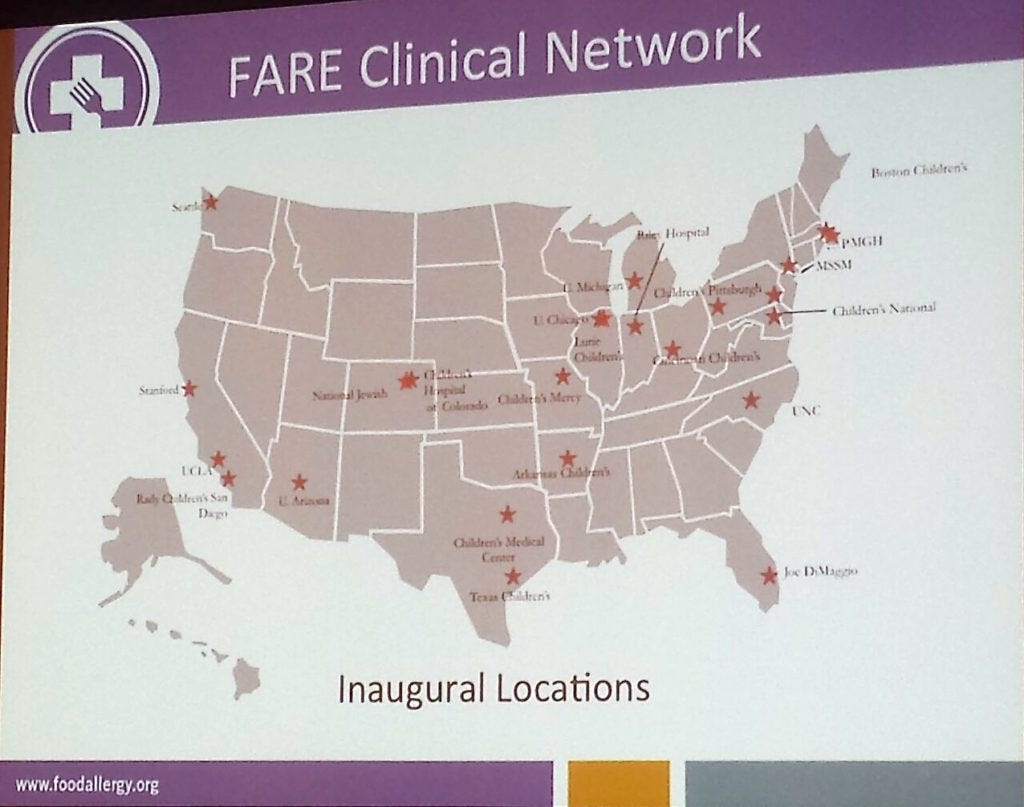
Here are the findings on Oral Immunotherapy (OIT) on these other foods:
Results of Oral Immunotherapy (OIT):
- Efficacy: OIT can induce desensitization and as such continued treatment is required. OIT has not been shown to create long-term tolerance.
- Safety: there are significant systemic side effects occur.
- Challenges: there are high rates of discontinued treatment with 10–20% of patients who are unable to tolerate OIT.
SLIT (Sublingual Immunotherapy)
- Efficacy levels are modest with 10% of patients achieving sustained unresponsiveness.
- Safety: SLIT appears safe.
- Challenges: there is a high rate of discontinued treatment as patients find difficulty in maintaining a daily dose.
EPIT (Epicutaneous Immunotherapy). — eg. Peanut Patch.
- Efficacy: EPIT may induce some level of desensitization.
- Safety: there is evidence that local site reactions are common.
- Unknowns: the magnitude, durability and long-term outcomes of EPIT are unknown.
In Summary:

Dr. Sriaroon went on to make the following statements regarding Future Directions:
- Development of a multi-allergen OIT.
- Developing next generation OIT/SLIT.
- Other approaches such as biological agents, DNA vaccines, Immunomodulating drugs, and Nanotechnology will be assessed.
Here is a list of drugs in phase 1/II and III trials that are currently being conducted.
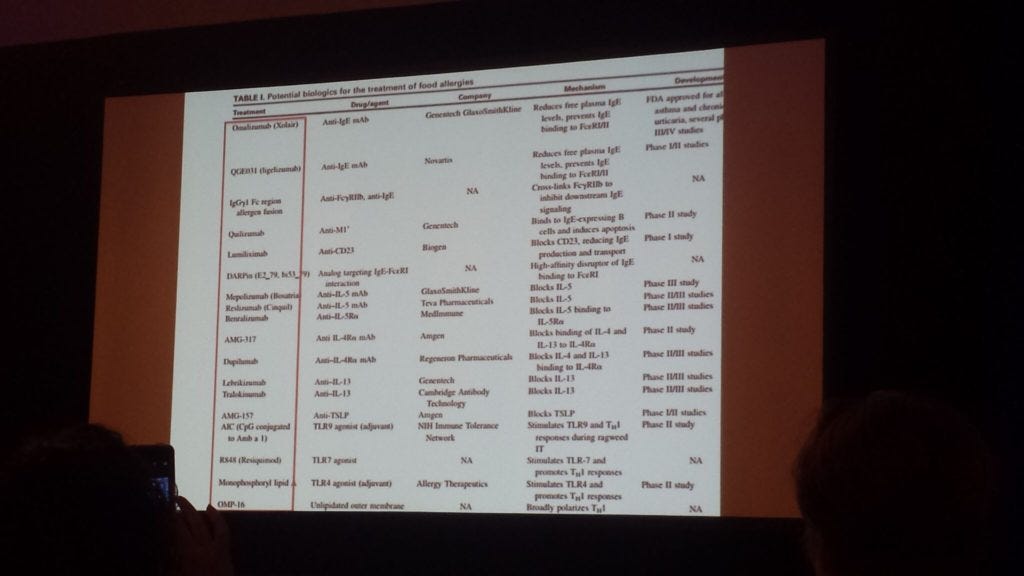
In Summary, finding a cure for food allergies may seem light years away, but compared to where we were even 5 years ago, there are some promising research studies in the pipeline. Strategically executed steps are being put in to place by F.A.R.E together with investor interest and a market for epinephrine are driving research demands. to enable better access of patients to clinical trials, eventually paving the way for FDA approved new treatments.
Below are some of the advocates, researchers, and clinicians working with food allergies in their daily lives.

Dr. Baker has been advocating to expand sites for food allergy clinical trials. Recently, with the Obama initiative, FARE was awarded $250,000 to start a food allergy research program that is patient centric within the next 2 years, starting May 1st, 2016. This does not seem like a lot of money, and it’s not, considering we are talking about a health issue effecting so many children.
Secondly, creating new drugs is great, but why isn’t more funding being put towards finding out what is causing food allergies? Considering that it has mysteriously grown exponentially in the last 20 years. According to allergist Dr. Moustafa who spoke later today, only 3–4% of people have a true food allergy. Yet 1 in 13 kids in classrooms around the United States are said to have food allergies. Dr. Mustafa says that this high range is driven by physicians “broad testing”. Dr. Moustafa’s opinion is that only foods that you are suspicious of, should be tested for. Dr. Sriaroon, who spoke this morning skipped right past the question of what is causing food allergies, because no one really has an answer for that. Yet.
One thing I’ve learned is that there are so many variables, motives and unknowns involved. Food for thought indeed.

Very sweet, knowledgeable and funny MD’s who have first hand experience with their own kids with food allergies.